A
reaction mechanism is a detailed step-by-step description of how reactants are
converted to products. It consists of a sequence of steps showing the making of bonds and the breaking of bonds. It is a way to
explain or rationalize how a reaction happens to change a reactant to a product.
When
bonds are made or broken, there are movements
of electrons from one molecule or a group of atoms to another.
In
most reactions you meet in organic chemistry (except radical reactions), the
electrons move in a pair (two). We draw the flow of this pair of
electrons as a curved arrow like
this:
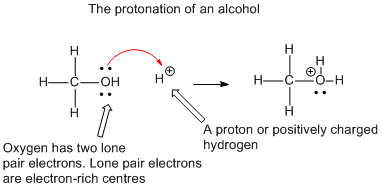
Another
important point to note is that these two electrons always move from a place of
more electrons (or negatively charged) to a place with less electrons (positively charged):
Here
are some common examples, which you meet in reactions you have learned:
Whichever a reaction may be, when drawing a mechanism, remember that a curved arrow means the flow of two electrons, and the electrons always flow from a place of more electrons to a place of less electrons, never the opposite direction.




No comments:
Post a Comment