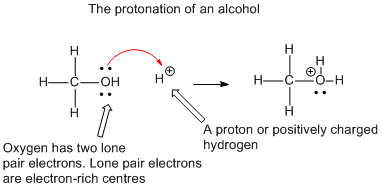
Dear
Students,
The
recent quiz question requires you to choose the correct compound between two
compounds, C and D, to which a given IR spectrum belongs to:
Let
us examine and compare them. Both are aldehydes (having CHO groups) and have
aromatic rings. The difference between them is compound C has a C=C while D does not. And this one difference makes for a lot of differences between the IR spectra given off by C
and D. Let us look at the spectrum.
 |
| IR spectrum given in the quiz |
Having
this double bond means C is a fully unsaturated compound: all the carbons in the molecules are sp2 carbons.
Looking at the 3000 cm-1 region, we see sharp, moderately intense
bands to the left of 3000 cm-1 (3062, 3083), C-H stretching
vibrations of sp2 or unsaturated carbons. No bands are seen to the
right of 3000 cm-1 except for a pair of bands (2826, 2724) which are stretching vibrations of C-H of the CHO group. This clearly shows that this is a spectrum
of an unsaturated compound. So this spectrum belongs to C.
Other
IR features that set the two compounds apart is the position of the sharp and
strong C=O stretch band. This C=O stretching vibration is at 1678 cm-1,
rather low (<1700). This lowered band frequency could be due to conjugation
of the carbonyl to a double bond and the aromatic system, and this is indeed
seen in compound C. Additionally, there is the presence of the C=C stretch band
at 1615 cm-1.
Now,
shown below is an actual spectrum of compound D. You can see many differences
if you compare it with that of C.